Our commitment to quality, at Bharat Serums and Vaccines, results from our vision and determination to provide the best possible products to our customers. We dedicate ourselves to quality by procuring raw materials from leading manufacturers and testing products in a cGMP compliance facility equipped with sophisticated equipment and systems.
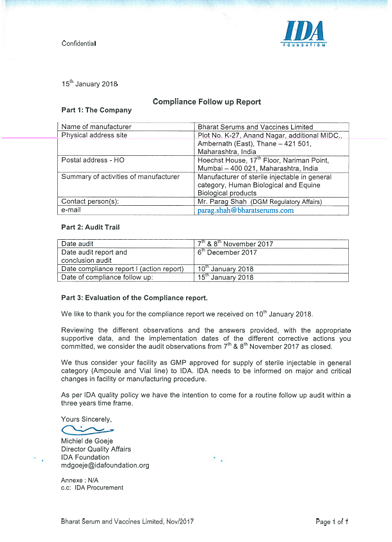
The QA team conducts regular internal audits to ensure that our operations adhere to standards set forth by regulatory bodies. The team also works closely with R&D to ensure that they are developing and testing new products following the principles of Quality by Design (QbD).
Our no-compromise approach to quality has helped us meet our commitment to improving and saving lives. We believe in going beyond the common methodology of merely adhering to regulatory standards.
Instead, we have developed a culture of quality compliance that ensures our stewardship in healthcare. We have fostered a like-minded team that regards quality as a way of life. Not surprisingly, the key tenets of quality and compliance are upheld in each of our functions.
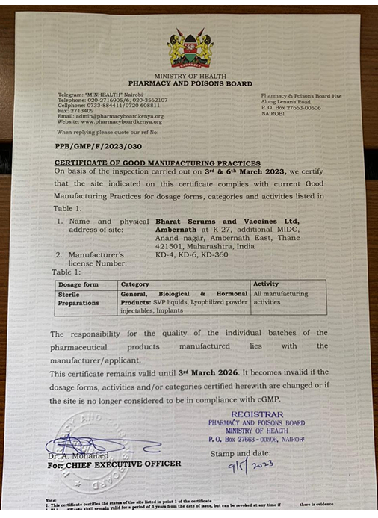
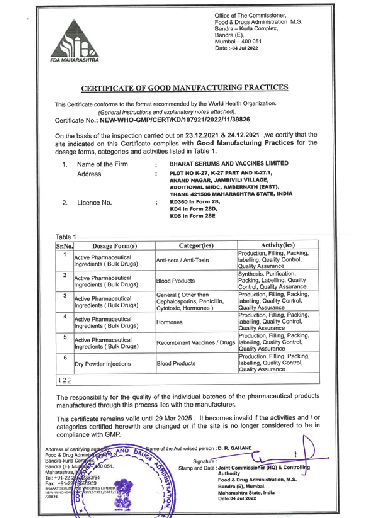
We constantly work to build quality into the product at every stage of production. This includes procuring raw materials from approved primary sources, manufacturing and packaging in a cGMP (Current Good Manufacturing Practice) compliant facility. The facility has cutting-edge machinery and systems for storing and distributing goods at appropriate temperatures. It further enables product disposal safely per applicable regulatory standards. Our manufacturing facility has a quality control laboratory that tests starting materials and finished products to ensure that we provide goods that meet all relevant quality requirements and standards. Our goal is to develop a quality management system that satisfies all applicable regulations as well as the standards set by regulatory authorities like CDSCO, EU GMP, ANVISA, PIC/s, ISO 9001:2015, and WHO amongst others.
Our quality team is brisk and centred, always ensuring that our risk-based system implementation and its routine verification. This is carried out through an impeccable internal auditing system to ensure that we stand by our promise of delivering world-class quality products to our customers.
Under the QbD (Quality-by-Design) principles, our Development Quality Assurance team collaborates with the R&D team to monitor quality from the developmental stage and appropriate implementation of new technologies.
We at BSV, are equipped with a cutting-edge system for validation and qualification of manufacturing facilities to ensure the quality remains uncompromised in every product. The process of initiating material vendor qualification and recurrent inspection of authorised stockists ensures the upkeep of quality standards throughout the supply and procurement chain.